Question #55e37
2 Answers
Feb 8, 2018
Explanation:
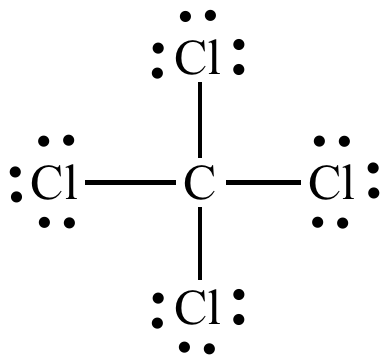
Electrons are shared between the carbon atom and each chlorine atom.
Feb 9, 2018
Explanation:
A simple molecule that is covalently bonded with chlorine in it can be
At room temperature, this molecule is a gas.
It is non-polar, as it has no difference in electronegativity between the two chlorine atoms.
Its structure looks like this:
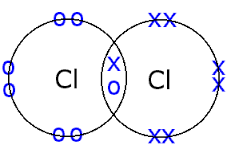
As you can see here, there is a covalent bond with the two atoms.


