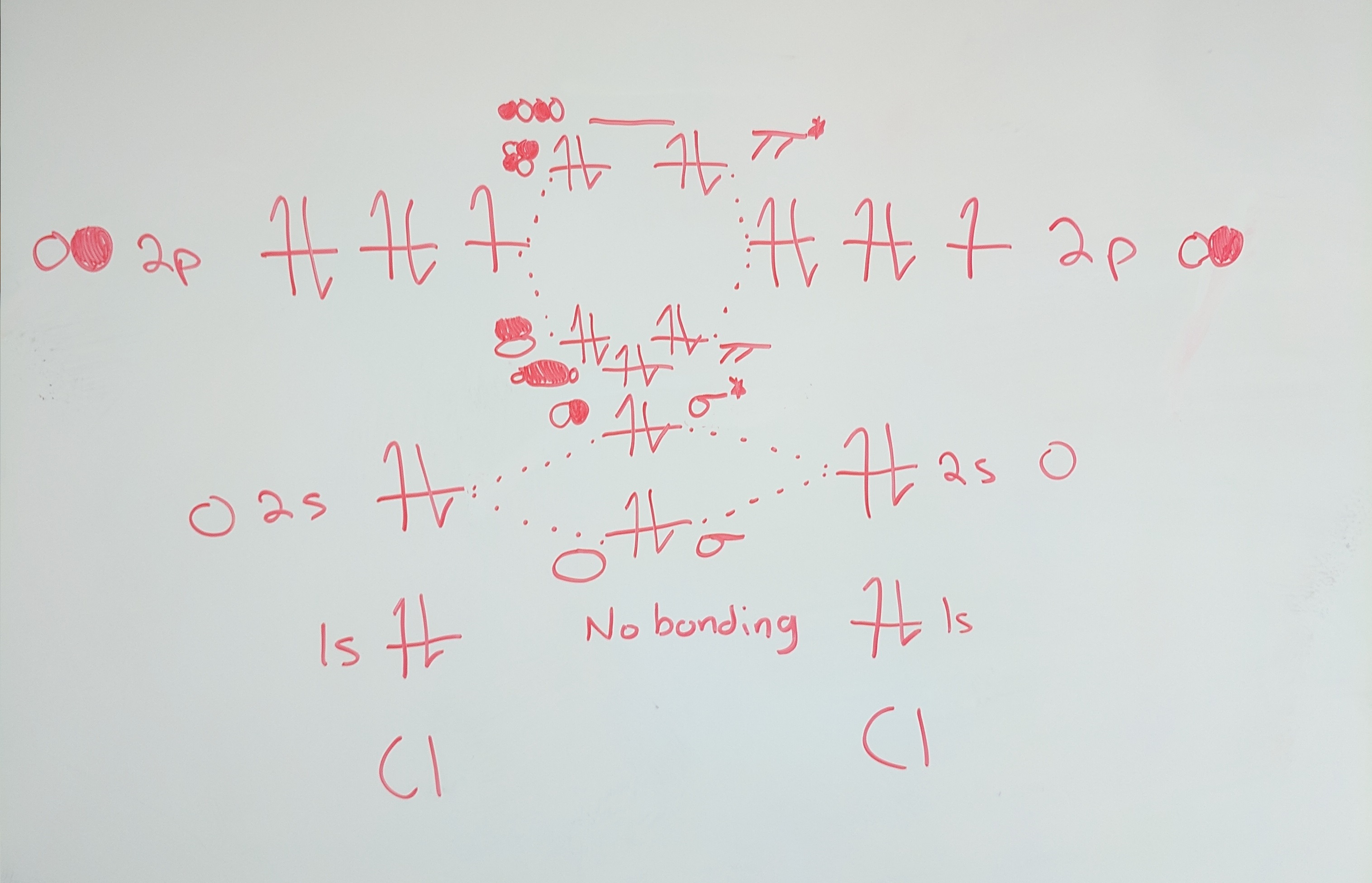The molecular orbital diagram for #Cl_2# is above. Since the 1s orbitals are far apart, they will not interact. Each 2s and 2p orbital will interact with the one of the other atom. #sigma# bonding will result from the s orbitals and #pi# bonding will result from the p orbitals. The bond order is (number of bonded electrons-number of antibonded electrons)/2, which is (8-6)/2=1.