Question #95e91
1 Answer
Feb 12, 2018
- Rutherford didn't know about the properties of electrons.
- He also didn't know why electrons didn't collapse into the nucleus.
Explanation:
This is Rutherford's model:
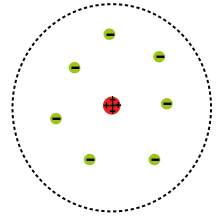
As you can see, Rutherford showed that there was a positively charged nucleus containing protons, with negatively charged electrons outside of the nucleus.
Rutherford also proposed that the electrons existed in an electron cloud—basically, a region of space outside of the nucleus.
However, he didn't understand how exactly they were moving.
He also didn't know why the negatively charged electrons didn't collapse into the positively charged nucleus due to electrostatic attraction, causing the atom to collapse.
Here's an awesome answer that goes into detail about how Rutherford came up with this model! :)

