Question #4d7ed
1 Answer
Feb 13, 2018
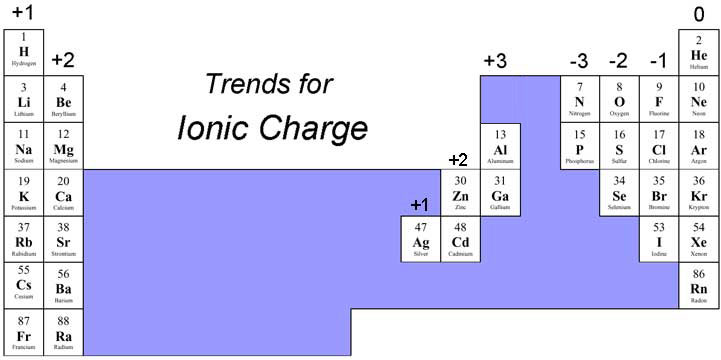
No, but knowing trends is key!
Explanation:
While it is useful to keep in mind the relative charges and formal charges of elements, you do not need to memorize these values.
By paying attention to the number of electrons on an element you can determine their respective oxidative states and are able to quickly identify overall net charges.
Al2O3 for example
We have 2 Al's which tend to enjoy a 3+ oxidative state, which is attached to Oxygens which prefer a 2- oxidative state.
2xAl (3+) = +6
3xO (2-) = -6
Therefore the net charge is 0