Why does capillary action occur?
1 Answer
Capillary action happens because the adhesion force is more stronger then the cohesion force.
Explanation:
Capillary action is an effect caused by the interactions of a liquid [e.g. water] with the walls of a tube, eg glass tube.
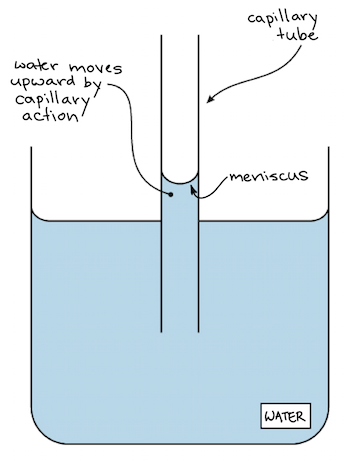
Water climbs up, aginst gravity, in a glass tube because of the strong hydrogen-bonding interactions between
On the video below is this process explained:
https://www.youtube.com/watch?time_continue=3&v=eQXGpturk3A
The capillary action depends on adhesion [attraction of molecules of one kind to molecules of a different kind] which enables water to go upwards through thin glass tubes and cohesion [attraction of molecules to other molecules of the same kind] interactions between water molecules.
In this case, addhesion forces are much stronger than the cohesion forces so water can move upward in a capillay tube.


