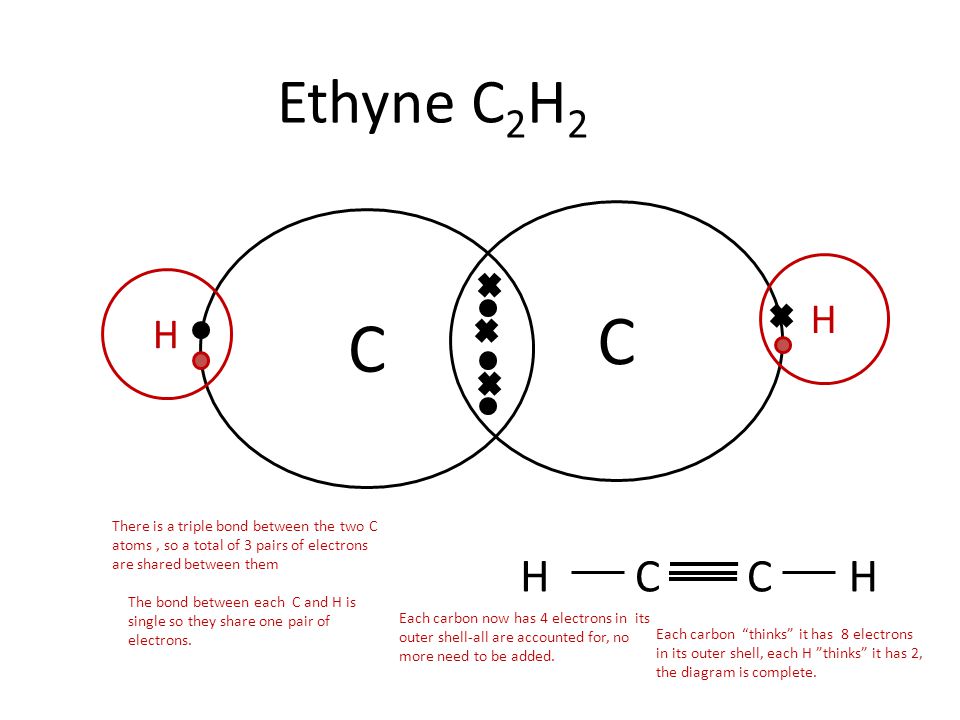
Question #05ab5
1 Answer
Covalent bonds are formed through the sharing of electrons between two non-metals.
Explanation:
There are two types of covalent bonds: polar and non-polar.
A polar molecule has an unequal sharing of electrons, which results in a partially positively charged end and a partially negatively charged end. This happens when two atoms have an electronegativity difference of greater than
A non-polar molecule has an equal sharing of electrons. This means that the molecule does not have charged ends. This happens when the two atoms in the covalent bond have similar electronegativities, or an electronegativity difference smaller than
In
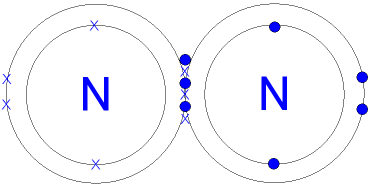
In
In