What is the rate of diffusion affected by concentration ?
1 Answer
Greater the difference in concentration, faster the rate of diffusion and vice versa.
Explanation:
Diffusion is the movement of material from the area where they are in higher concentration to the area where they are in lower concentration without any expenditure of energy.
Now we can see from definition that it's the transport which basically occurs due to difference in concentrations of particles in two environments. If one area, suppose area
Now, let's suppose, there is not much difference in the concentration of both areas. Area
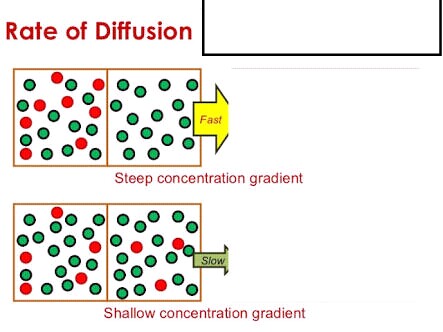
Hope it helps!

