Why are low molecular weight alcohols soluble in water? And why does the water solubility of alcohols decrease with the increasing size of the hydrocarbon group? Can you explain this?
1 Answer
Well, what is acting on the alcohol when it is dissolved in water....?
Explanation:
Both, say methanol, and ethanol, are water-like solvents...they are in effect HALF a water molecule..and reasonably, both alcohols are infinitely miscible with water. Methanol, in particular, is INSOLUBLE in hexanes. The influence of the hydroxyl group confers this water solubility and hexanes insolubility.
As the hydrocarbyl tail GROWS, the solubility of the alcohol in water decreases. Propanol is the cut-off point; butanol, and the higher alcohols exhibit limited water solubility. For longer chain alcohols, non-polar interaction between the hydrocarbyl chains becomes more significant as an intermolecular force.
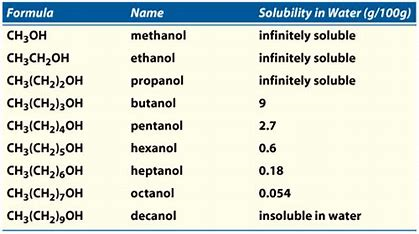
Are the results in the Table consistent with what we have argued? Why or why not? How do you think the solubility of isomeric alcohols would compare? Say

