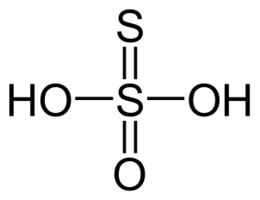
What is the empirical formula of a compound comprised of 1.75% hydrogen, 56.1% sulfur and 42.15% oxygen?
1 Answer
The empirical formula for this compound is:
This compound is thiosulfuric acid.
Explanation:
An empirical formula represents the smallest whole number ratio of elements in a compound.
Since the percentages add up to
The steps for determining an empirical formula are:
1) Determine the moles of each element from the masses given and their molar masses.
2) Find the mole ratio for each element by dividing moles of each element by the smallest number of moles.
3) If the mole ratios are whole numbers, then they are the subscripts for each element.
4) If the mole ratios are not all whole numbers, multiply by a factor that will make them all whole numbers.
Moles of each element
Divide the mass in grams by the molar mass of each element. The molar mass is the element's atomic weight on the periodic table in
Mole ratios
Divide the moles of each element by the smallest number of moles, which is
Since the molar ratio for
The empirical formula for this compound is:
This compound is thiosulfuric acid.