Why Cu does not reacts with HCl?
1 Answer
Feb 25, 2018
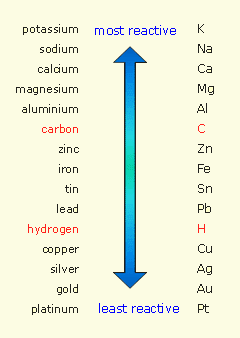 http://www.frankswebspace.org.uk/ScienceAndMaths/chemistry/reactivitySeries.htm
http://www.frankswebspace.org.uk/ScienceAndMaths/chemistry/reactivitySeries.htm
Copper is below hydrogen on the reactivity series.
Explanation:
Elements on the table at the top (most reactive) have a tendency to lose electrons more easily than their less reactive counterparts. The elements lower in the series (like gold) are electron "thieves", in that they can oxidize elements more easily.
Hydrogen can only react in displacement reactions with elements above it in this series.

