balanced chemical equation for ? #"Ni(NO"_3)_2 +"NaCO"_3"##rarr##"NaNO"_3 + "NiCO"_3"#
how I balance
how I balance
2 Answers
Explanation:
I believe in your equation, it should be
Balancing a chemical equation means to have the same kinds and amounts of atoms on each side of the equation. So that means there must be equal amounts of nickel, nitrate (
A strategy to balancing reactions is to start with one inequality and work from there.
For example, in the unbalanced equation:
there are two nitrate molecules in the reactants, but only one on the products. Double the whole
After you make a change, check to see how it affects the whole reaction. There are now 2
However, there are already 2
Check the number of nickel atoms. One on the reactants, one on the products. Check.
Check the number of carbonate molecules. One on reactants, one on products. Check.
Now that you checked all the atoms and molecules, the balanced equation is:
Explanation:
The formula for sodium carbonate is
Balance the following equation.
The down arrow means that the solid is a precipitate.
Both anions are polyatomic ions; the nitrate ion
Lets look at the cations first. We have
Is this equation balanced? Let's check how many of each ion are on each side of the equation.
Left-hand side
The numbers of each ion on both sides of the equation are the same now, so it is balanced.
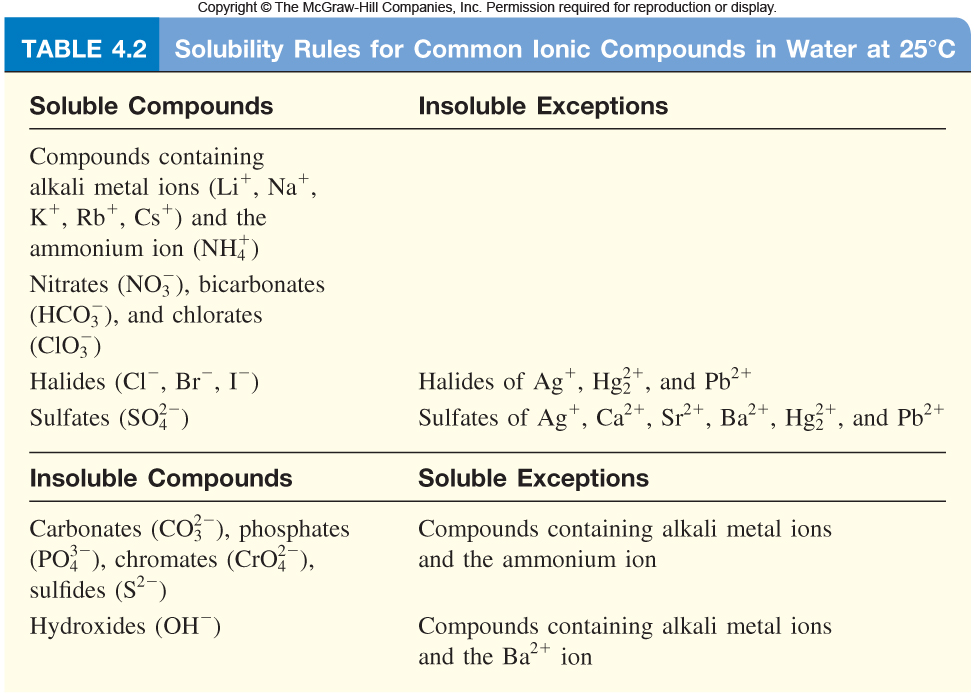
I am adding a table of solubility rules so you can see how I knew which compounds were soluble and insoluble.