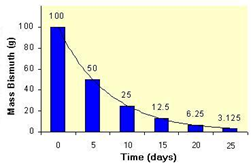
3. Below is the decay curve for bismuth-210. What is the half-life for the radioisotope? What percent of the isotope remains after 20 days? How many half-life periods have passed after 25 days? How many days would pass while 32 grams decayed to 8 grams?
1 Answer
See below
Explanation:
Firstly, to find the half-life from a decay curve, you must draw a horizontal line across from half of the initial activity (or mass of the radioisotope) and then draw a vertical line down from this point to the time axis.
In this case, the time for the mass of the radioisotope to halve is 5 days, so this is the half-life.
After 20 days, observe that only 6.25 grams remain. This is, quite simply, 6.25% of the original mass.
We worked out in part i) that the half-life is 5 days, so after 25 days,
Finally, for part iv), we are told that we start off with 32 grams. After 1 half life this will have halved to 16 grams, and after 2 half-lives this will have halved again to 8 grams. Hence, a total of 2 half-lives (that is, 10 days), will have passed.
You can model this quite simply by an equation like
Remaining Mass
where

