What can help a chemical reaction get started? A. A flame B. Mixing materials C.Placing materials on a hot plate D. All of above
2 Answers
D. All of the Above.
Explanation:
Reactions need the atoms or molecules involved to collide and react. The collisions have to be at the right energy levels and often at the correct angle of contact.
A. A flame will add energy to the reaction providing the energy of activation necessary for the reaction to start.
B. Mixing (stirring) the chemicals will increase the number of collisions. Not all of the molecules have the same amount of kinetic energy ( see the evaporation of a liquid at room temperature) A few of the collisions will have sufficient energy and the right angle of collision to react. If the reaction is exothermic, the few that react will release energy enabling other molecules to react, starting a chain reactions that gets the chemical reaction started.
C. The hot plate like the flame will add energy to the reactions providing the activation energy to start the reaction.
D. all of the above are correct.
D
A & C are ways of heating the chemicals to give them energy and B allows more molecules to "touch" and react.
Explanation:
Chemical reactions involve molecules re-arranging to form new products. Each molecule needs to touch another to form new bonds. They also need a certain amount of energy to change these bonds. [See image] This is known as Activation Energy.
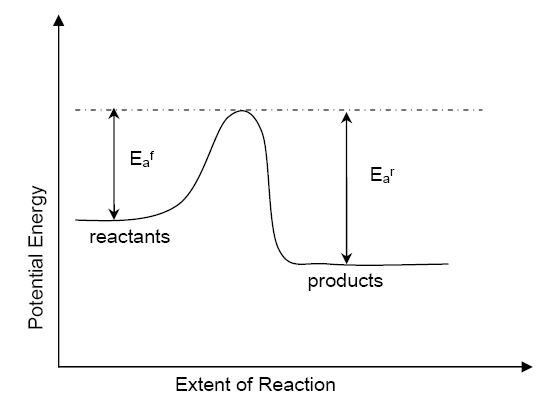
In general you can start a reaction by heating the reactants (which will give each molecule more energy, closer to the activation) and also mixing the reactants (so that each molecule has more chance of collision).
Hope that helps!

