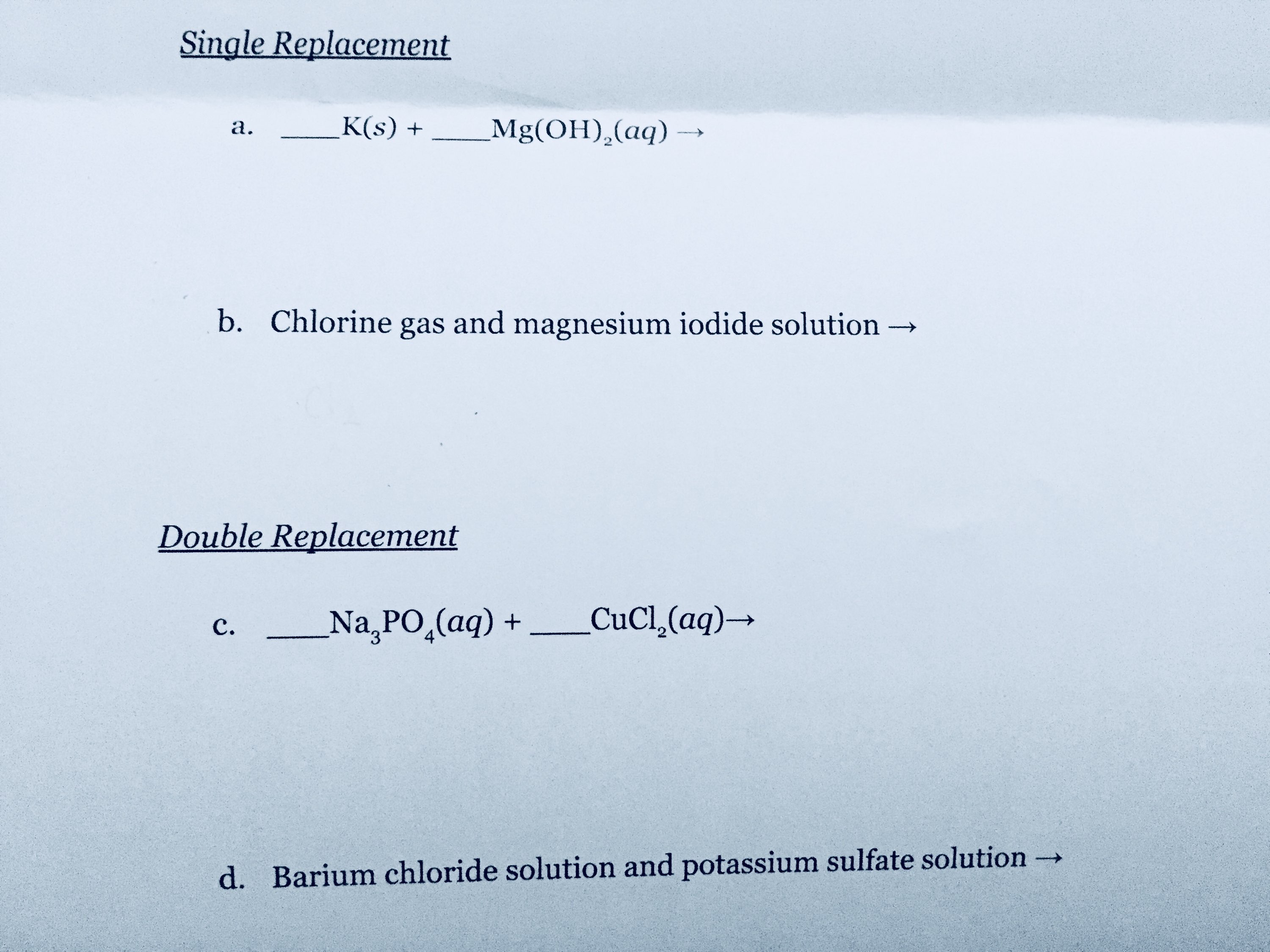
How do you predict the product of a reaction?
Please help me, this is my fourth time uploading this question with varying titles.

Please help me, this is my fourth time uploading this question with varying titles.
1 Answer
Mar 1, 2018
Explanation:
For single replacement, the rule is the more reactive metal replaces the less reactive cation in the aqueous solution, and for double replacement, a solid, liquid or gas must be formed.

