Which ion has the most shells that contain electrons??
1 Answer
Maybe the ion from the element Tennessine,
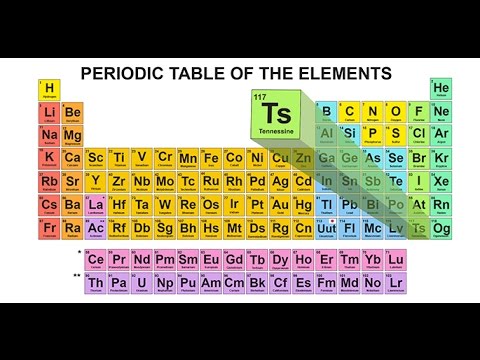
Explanation:

In 2016 IUPAC announced the new name tennessine (Ts) for element 117 in place of the temporary systematic name (Uus). As you can see
We can not for sure say that the teoretical ion
If an atoms gains electrons, it develops a negative charge and becomes an anion and his last shell contains more electrons [as seen on the example below with Fluorine,
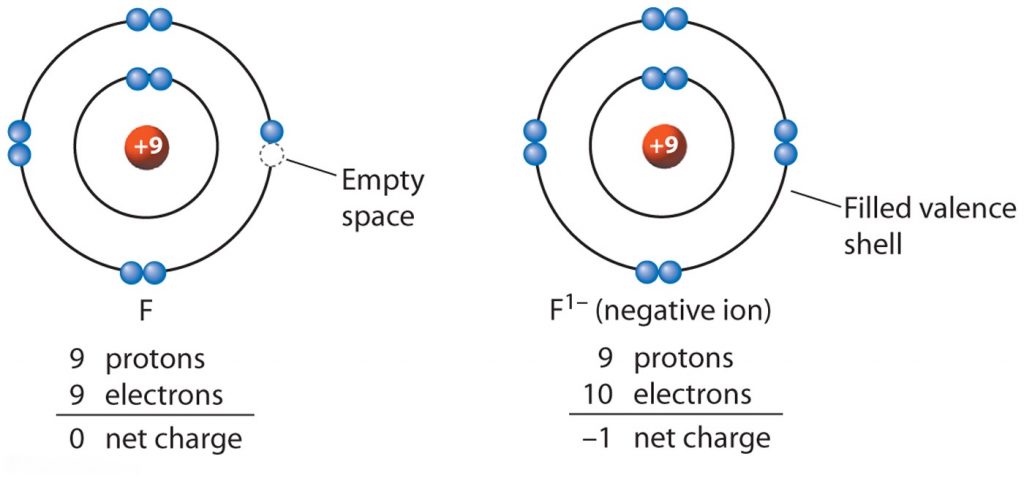
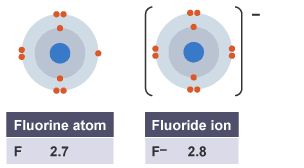
If the element
In the last shell is the gained electron circled with the blue "marker". Now the ion
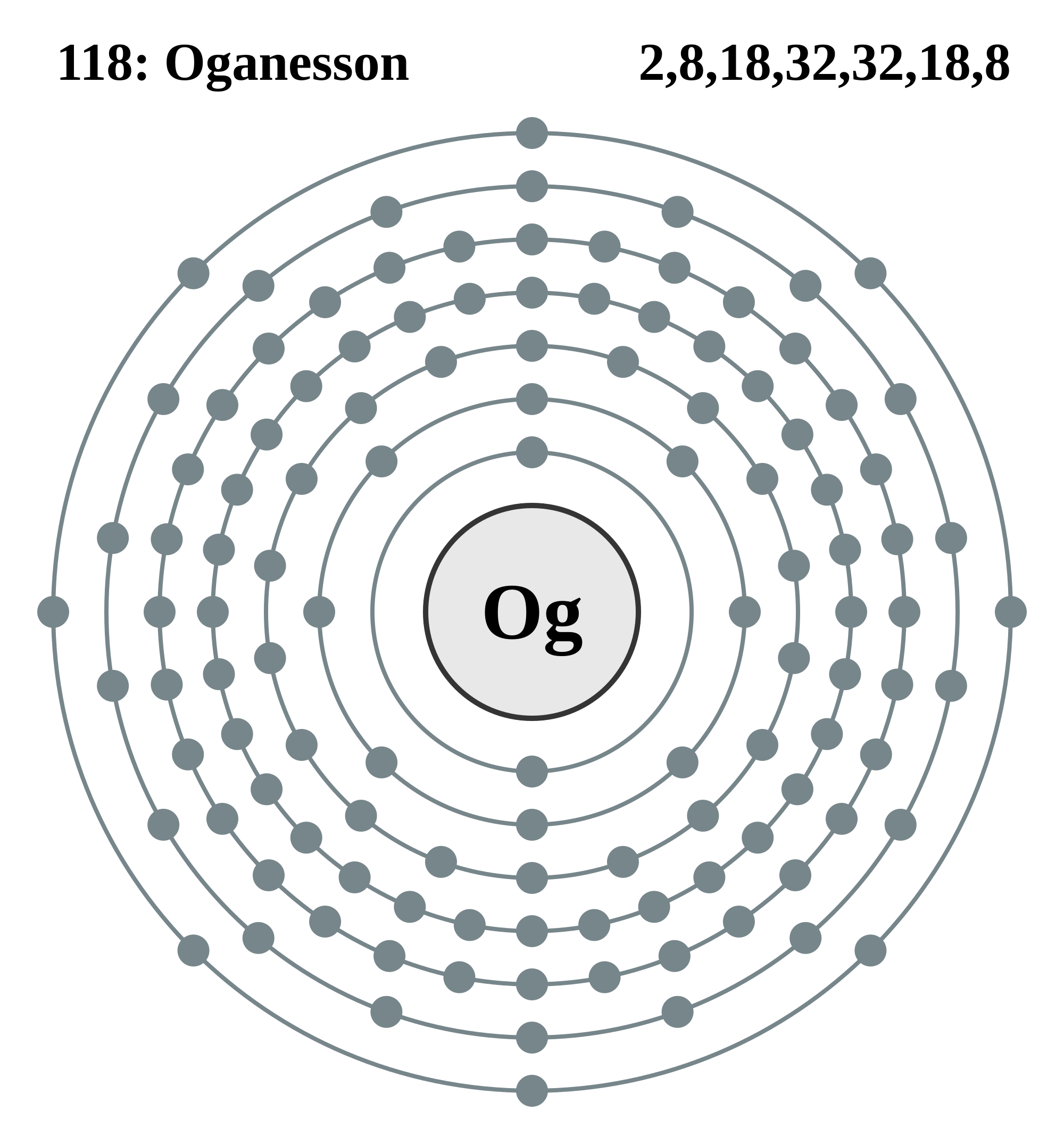
You may have noticed that I have used the word "maybe" and "probably" in my answer and explanation. This is because the "new" element has predicted properties, as seen below.

