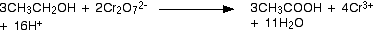What happens during the incomplete oxidation of alcohol?
Provide the answer by stating the conversion of alcohol into aldehyde, ketone and finally to carboxylic acid.
Provide the answer by stating the conversion of alcohol into aldehyde, ketone and finally to carboxylic acid.
2 Answers
You have outlined the steps....we could take a leaf from inorganic chemistry, and assign oxidation states... We use ethane and ethanol as examples....
Explanation:
And ethyl alcohol is oxidized to acetaldehyde...;
And acetaldehyde is oxidized to a carboxylate...;
And for a ketone, clearly accessed from say isopropyl alcohol, whose ipso carbon is clearly zerovalent, we would write...
And often we speak of the oxidation of the alcohols to carbonyls, and thence to carboxylic acids...of course the carbon could be oxidized even further....to what?
Just to add, that most of the time, we use acidified dichromate or permanganate as oxidants. These are fairly ferocious and indiscriminate, tho they get the job done... A range of selective oxidants are now available
This is all about complete and incomplete oxidation.
Explanation:
The oxidising agents commonly used are Potassium Dichromate or Sodium dichromate.
We get an aldehyde when we use an excess of the alcohol, and distil off the aldehyde as soon as it forms.
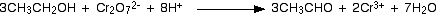
or much simple you can represent it as:-

Now the full oxidation of alcohols will form carboxylic acids.