What is the molar mass of adrenaline (C9H13NO3) ?
1 Answer
Mar 7, 2018
Explanation:
Molar mass just means the mass of
So, the molar mass of
In turn,
#9# moles of carbon.#13# moles of hydrogen.#1# mole of nitrogen.#3# moles of oxygen.
This tells us that:
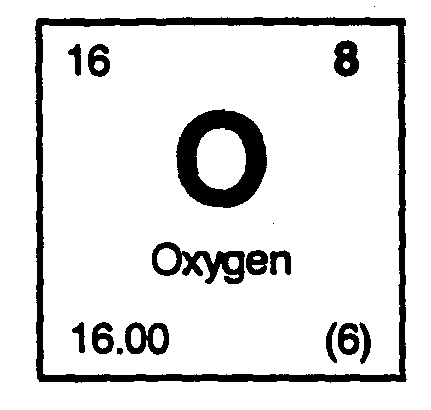
From the periodic table, we can find the mass of

