How is hydrogen bonding similar to dispersion forces?
1 Answer
They're both dipole-dipole intermolecular forces (but that's where the similarity ends).
Explanation:
In hydrogen bonding, which occurs only in molecules with
The slightly positive dipole, or center of positive charge, will be attracted to the slightly negative dipole of another molecule.
This attraction is called a hydrogen bond, and holds different molecules together.
Therefore, due to it both holding together different molecules and arise from attraction between dipoles, it is a dipole-dipole intermolecular force.
Here's a water molecule to demonstrate this:
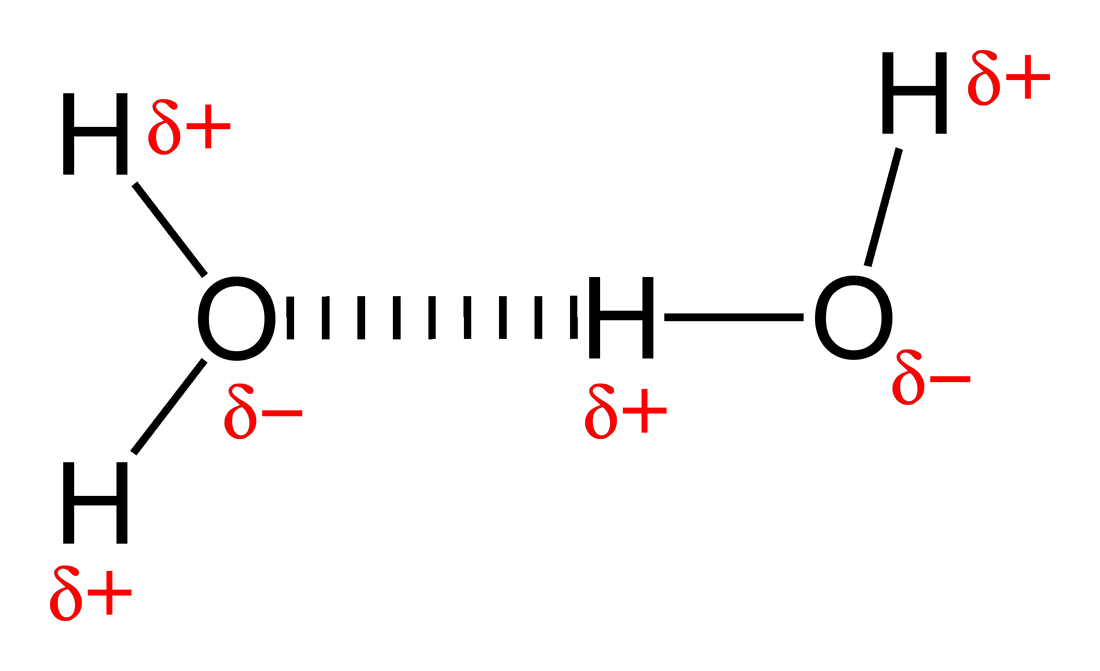
The dotted line represents the hydrogen bond.
However, intermolecular forces can also exist in nonpolar molecules, where dipoles don't exist. This is how it happens:
Electron movement is random, so when the electrons of one molecule or atom happen to be more concentrated towards one side, that side becomes very slightly negative and the other side becomes very slightly positive.
An instantaneous dipole is created.
This causes an induced dipole in another molecule or atom, which in turn causes a short-lived attraction between the two molecules or atoms.
Here's a visual to illustrate:
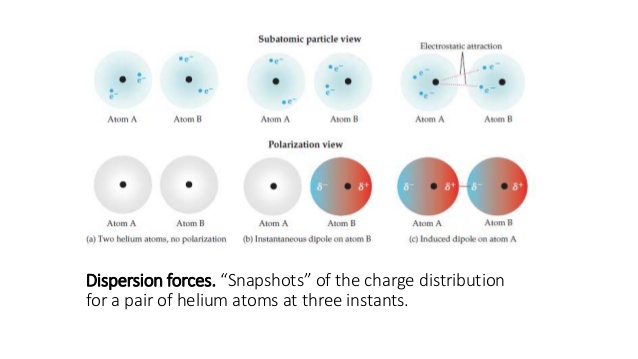
These forces are called London dispersion forces. Because they also result from attractions between dipoles and hold together molecules or atoms, they are considered dipole-dipole forces.
Although they are, more correctly, considered induced dipole-induced dipole forces, they are nonetheless still dipoles.
That's where their similarity with hydrogen bonding ends, though—hydrogen bonds are much stronger than London dispersion forces.

