Sir, How I Explain the chemical bonding of Hydrogen Fluoride (HF)?
1 Answer
Mar 14, 2018
Hydrogen fluoride is a covalently bonded molecule
Explanation:
Definition: Fluorine is the most electronegative element on the table 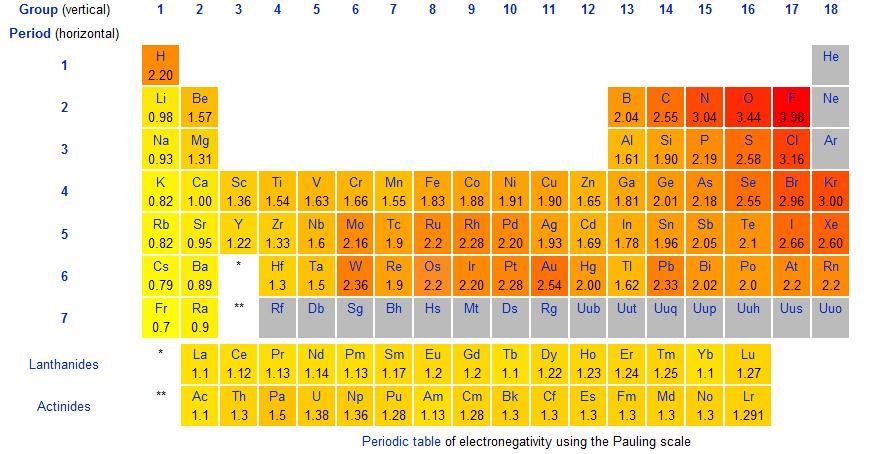
Interpretation: Fluorine bonds very strongly to hydrogen because it wants to fill its valence shell through a bond. It must be a covalent bond because Hydrogen is already very stable and would be unfavorable if it was taken off completely (in an ionic bond).
In order, it goes HF>HCl>HBr>HI in terms of intramolecular bond strength.
HF is not an ionic bond because by definition, ionic bonds are electron sharing between a metal and nonmetal.