What would you do to a question like this one?
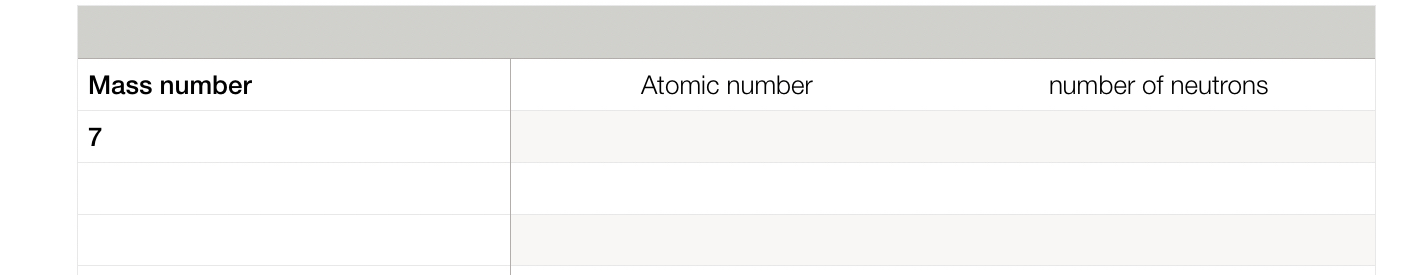
What is the atomic number and the number of neutrons?

What is the atomic number and the number of neutrons?
2 Answers
You simply DON'T KNOW here...
Explanation:
The mass number of an atom is to a first APPROXIMATION the SUM of the massive nucular particles, protons, and neutrons. Now it is a fact that
The question is admittedly poor...and requires better specification.
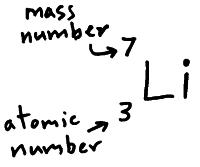 mass number is show in the pic
mass number is show in the pic
Explanation:
1- Mass number is always the larger number
2- Atomic number is the small one
so if mass number is 7 go to the periodic table and search for number 7 is you find it make sure its the larger number of the element
the answer is :
mass number is : 7
this element is Li (lithium)
so the Atomic number is 3
neutron = mass no. - atomic no.
neutron = 7-3 = 4
Atomic number equal to the number of P+ and equal to the number of e-
- note : remember atomic number is the small one.
- atomic number = proton = electron
to find neutron subtract mass number from atomic number to find the neutron :
remember this formula to find the neutron :
neutron = mass number - atomic number
if you have any question feel free to ask me
and hope you understand,
Amna.

