An explanation of why an ionic bond is solid at room temperature?
1 Answer
Well, an ionic compound is usually a solid at room temperature... not an ionic bond.
Common examples are
Their high melting points are due to strong interatomic ion-ion interactions in a three-dimensional lattice structure... These forces are due to the interaction of one ion with many other counterions, and depend on both size and charge (charge being a more significant factor).
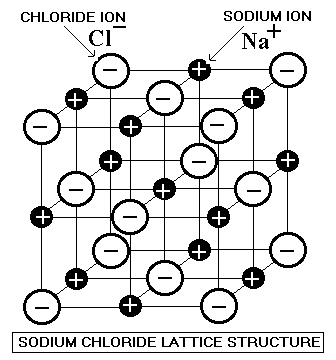
Obviously
As a result, these substances cannot melt... having less than enough thermal energy to melt, they must be less energetic than liquids. Therefore, they must be solids.
Can you propose an ionic compound with a melting point higher than

