Balmer series says transitions from n ≥ 3 to n = 2 as n= 2 is the ground state ? how Hydrogen atom electron gain energy from level 2 to higher levels as normally H electrons in level n = 1 ?
it does not transit from n 2 to n 3 when electron come back from n 3 there is two possible ways drop from 3 to 2 then to 1
and the other drop from 3 to 1
it does not transit from n 2 to n 3 when electron come back from n 3 there is two possible ways drop from 3 to 2 then to 1
and the other drop from 3 to 1
1 Answer
The Balmer series considers transitions that END at
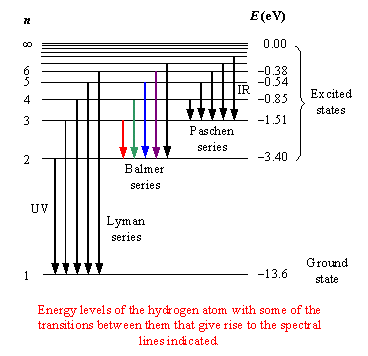
The ground state is and always will be
Any ensemble of hydrogen atoms will generate a spectrum consisting of all of the above (Lyman, Balmer, Paschen, Brackett series).
Some INDIVIDUAL hydrogen atoms will exhibit Lyman lines at one moment in time as they decay to the ground state, some other INDIVIDUAL atoms will exhibit Balmer lines as they decay to the first excited state, etc.
What we observe then is the collective spectrum for the ensemble at once.

