Please solve this electrophile addition based reaction problem?
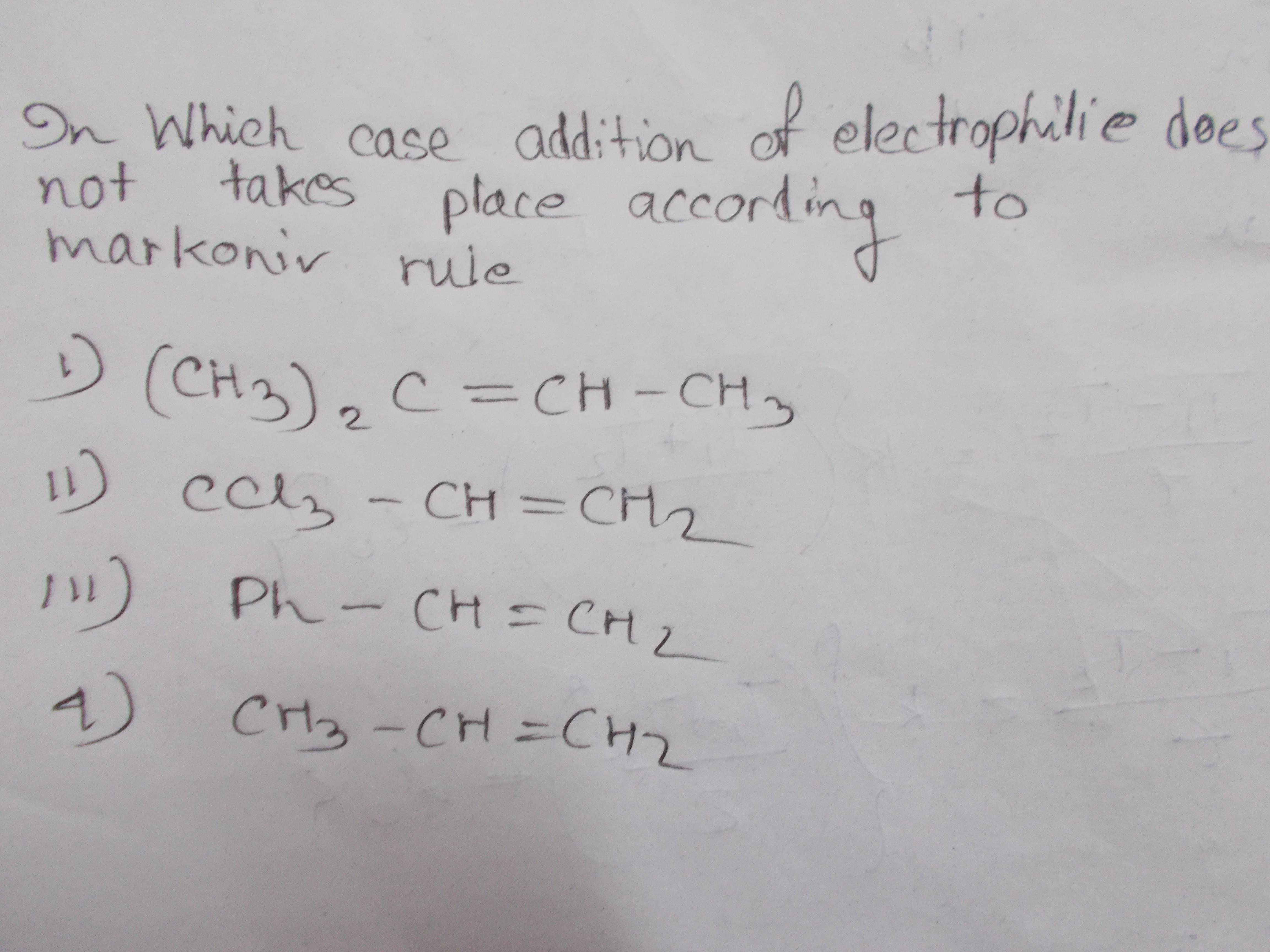
1 Answer
Mar 18, 2018
It's the one that tries to place electronegative atoms next to the cationic carbon. Why would a positive region want to give away electron density it does not have?
Markovnikov addition would be that the proton is added onto the carbon in a double bond that had more hydrogen atoms to begin with.
That would be disfavored if there is an alternate route that forms a more stable carbocation than the one that would be formed in the usual route. In this case we are looking for the Markovnikov route that gives the LEAST stable carbocation...
Draw out the first step in the electrophilic addition mechanism of some
- The tertiary carbocation is one of the most stable, as it has three
#R# groups around it with#"C"-"H"# bonds to spread electron density into the empty#p# orbital. - The secondary carbocation with
#"CCl"_3# adjacent to it is DESTABILIZED as all three#"Cl"# atoms are highly electronegative, withdrawing electron density away from a positive region... - The carbocation adjacent to the phenyl group is stabilized by resonance, spreading the positive charge around the ring.
- The secondary carbocation is less stable than the tertiary carbocation (one less
#R# group), but still has hyperconjugation going on which does introduce stabilization.

