What does ligand field theory enables us to explain that crystal field theory does not?
a. Low and high spin complexes
b. Colours of transition metal compounds
c. Stabilities of complexes
d. The spectrochemical series
a. Low and high spin complexes
b. Colours of transition metal compounds
c. Stabilities of complexes
d. The spectrochemical series
1 Answer
The spectrochemical series. Those are ordered from lowest to highest based on
Low spin and high spin can be "explained" on the basis of electron repulsions, colors can be explained based on the size of the crystal field splitting energy, and stabilities of complexes can be explained based on the way the orbitals are filled.
That is covered in more detail in these references:
The spectrochemical series is given in this answer, but would not be derived from Crystal Field Theory:
#overbrace("O"_2^(2-) < "I"^(-)< "Br"^(-) < "S"^(2-) < "SCN"^(-) "(S–bonded)" < "Cl"^(-) < "N"^(3-) < "F"^(-) < "NCO"^(-))^("mostly "pi" donors")#
#< overbrace("OH"^(-) < "C"_2"O"_4^(2-) ~~ "H"_2"O" < "NCS"^(-) "(N-bonded)" < "CH"_3"CN")^("mixed "pi" donors + "sigma" donors")#
#< overbrace("py" ("pyridine") < "NH"_3 < "en" ("ethylenediamine"))^("mostly "sigma" donors")#
#< overbrace("bipy" ("2,2'-bipyridine") < "phen" ("1,10-phenanthroline") < "NO"_2^(-) < "PPh"_3 < "CN"^(-) < "CO")^("mixed "sigma" donors + "pi" acceptors")#
The farther to the right you are in the series, the more strong-field the ligand field is, and the larger the ligand field splitting energy becomes. Therefore, the complex would be predicted to be low-spin if that is the case.
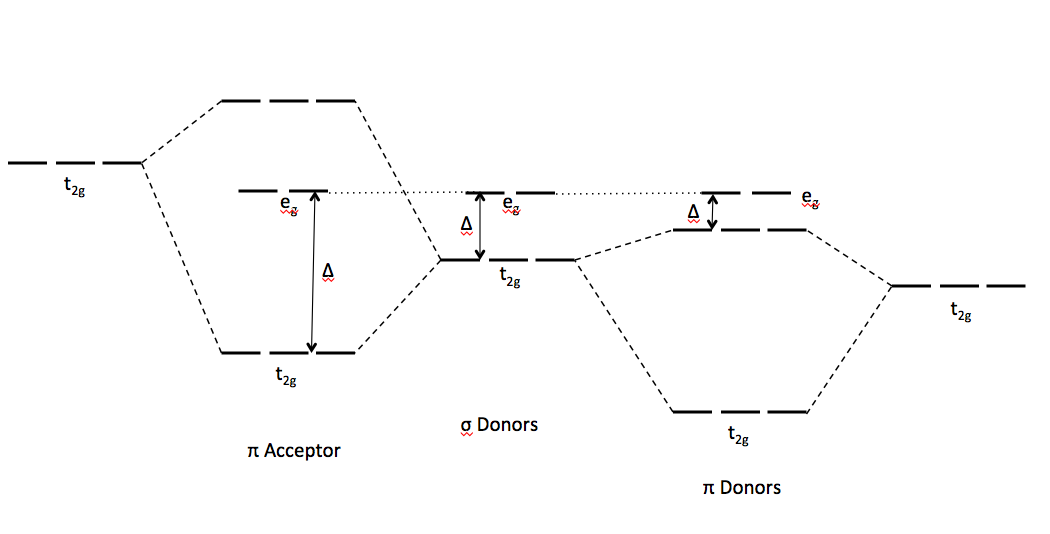
To predict this series, ligand field theory states that ligands come in with orbitals that interact with the metal orbitals. This is summarized below:
#pi# donors donate electron density from orbitals sidelong to make side-on interactions, slightly destabilizing#pi# -type orbitals (for example,#t_(2g)# orbitals in an octahedral field).#sigma# donors donate electron density from orbitals head-on to make head-on interactions, destabilizing#sigma# -type orbitals (for example,#e_g^"*"# orbitals in an octahedral field).#pi# acceptors accept electron density from orbitals sidelong to make side-on interactions, stabilizing#pi# -type orbitals (for example, the#t_(2g)# orbitals in an octahedral field).
And we can see that TYPICALLY, the ligand-field splitting energy is largest due to
And so, we order it so that the weakest influence to strongest influence is
#"pi donor" < "sigma donor" < "pi acceptor"#