What are the types of intermolecular forces acting in the liquid state of krypton? What are the ones acting in the liquid state of nitrogen fluoride?
1 Answer
Explanation:
(Assuming nitrogen fluoride refers to
In the liquid state of krypton (which would have to be at an extremely low temperature), the only intermolecular forces present would be London dispersion forces.
This is because krypton, being monatomic, is nonpolar.
The only intermolecular forces in nonpolar molecules are London dispersion forces.
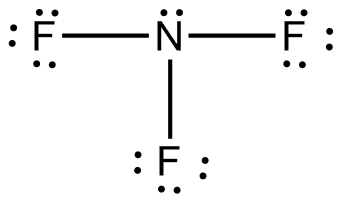
There is one lone pair around the central nitrogen atom, along with three bonds. So, the VSEPR shape of
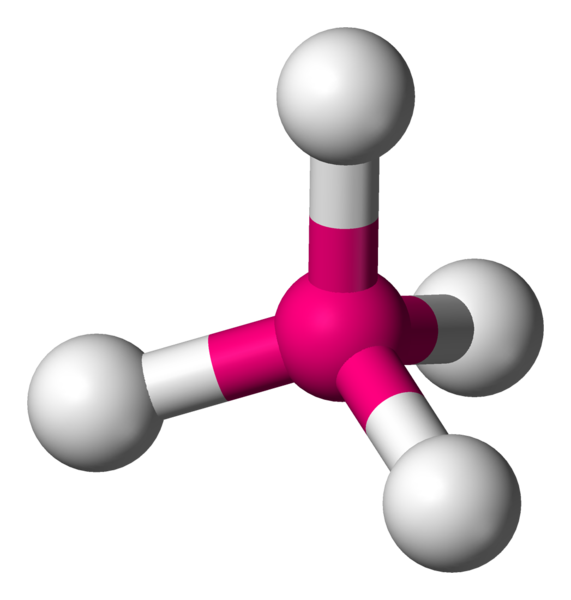
The lone pair will push the three bonded pairs, creating a slightly positive end and a slightly negative end.
The polarity of
There are no hydrogen bonds, because

