List the major types of intermolecular forces in order of in creasing strength. Is there some overlap?
1 Answer
London dispersion forces, dipole-dipole forces, and hydrogen bonds (overlaps with dipole-dipole forces).
Explanation:
- London dispersion forces.
London dispersion forces are the dominant intermolecular forces present in non-polar molecules, or molecules that don't have slightly positive and negative sides.
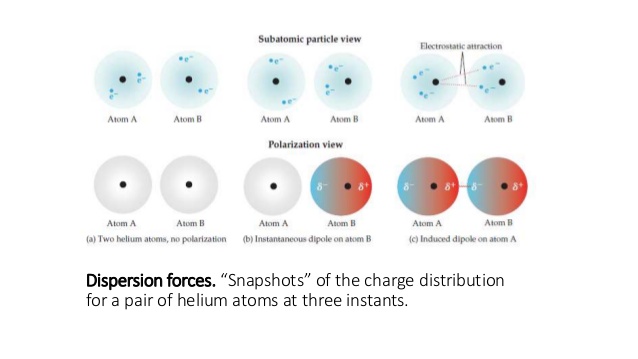
It happens when the random movement of electrons randomly causes one molecule to become polar (because there are more electrons on that side), which is called a spontaneous dipole.
The spontaneous dipole will then cause an induced dipole in a neighbouring molecule, creating a weak attraction—that attraction is called London dispersion forces.
- Dipole-dipole forces.
These forces are present in polar molecules, and happen when the positive end of one molecule is attracted to the negative end of another molecule.
They are stronger than the short-lasting London dispersion forces, because dispersion forces are the attractions between temporary dipoles and dipole-dipole forces are the attractions between permanent dipoles.
- Hydrogen bonding.
Hydrogen bonding is still the attraction between slightly positive and slightly negative sides, except the positive side is
It overlaps with dipole-dipole forces, because it is still the attraction between positive and negative dipoles.
But it is the strongest type of dipole-dipole forces, because hydrogen has a very low electronegativity (making its side very positive) and

