Why can CO_2 be easily solidified?
2 Answers
The process of making
Explanation:
In the dry ice industry (I hope there's one), before
In reality, there are two ways of making dry ice:
The cheaper and more efficient way is by pressurising and cooling
As the
The not-so-cheap but faster way is by increasing the pressure much higher than the above method and cooling the
As for your statement about why
It's not that easy to solidify it... In fact, I would say it is HARDER to solidify
Let's ACTUALLY look at the phase diagram:
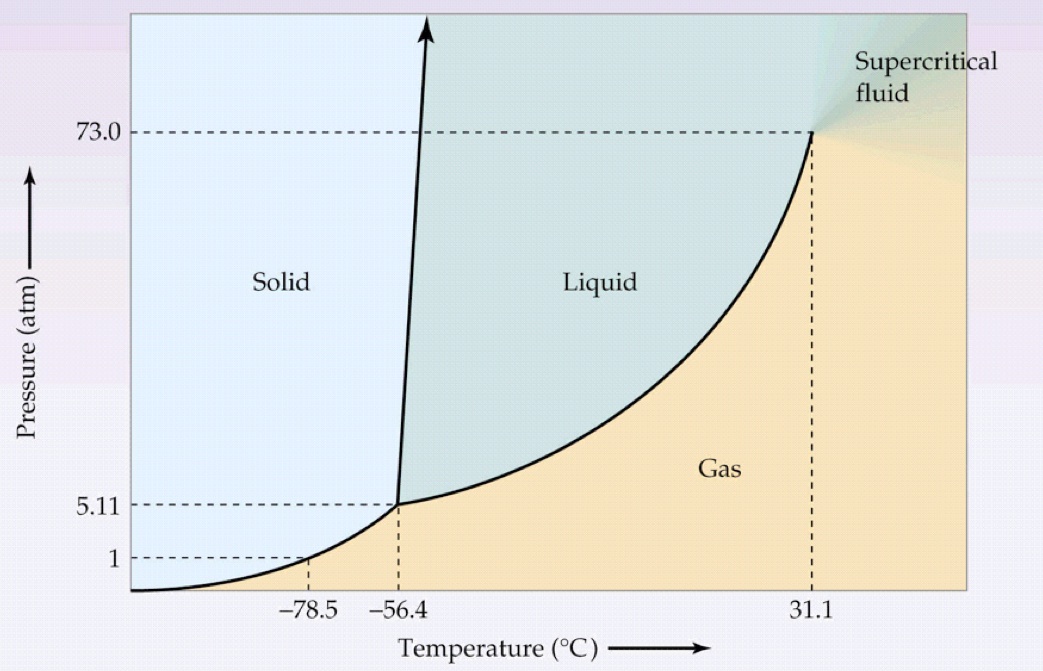 http://ch302.cm.utexas.edu
http://ch302.cm.utexas.edu
How dry ice is usually made is that it is pressurized into a liquid at typical cold temperatures (
 https://www.continentalcarbonic.com/
https://www.continentalcarbonic.com/
That means we first move UP the phase diagram from a gas to a liquid, and then LEFT from a liquid to a solid.
It's not to say that is easy... but after releasing the dry ice into normal atmospheric pressure conditions, it spontaneously will sublime, crossing the solid-gas coexistence curve rightwards at the