Describe a condition where we could find water in liquid form where temperatures exceed 100C?
This phase diagram is included: 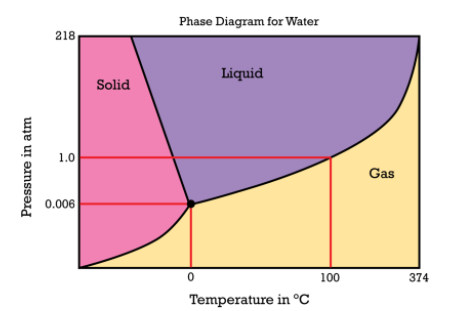
This phase diagram is included:
1 Answer
Apr 4, 2018
This would happen when the pressure is greater than 1 atm.
Explanation:
The boiling point of water is the traditional 100C, but that is only when the pressure over that water is 1 atm. If the pressure is increased, the boiling point is increased (as per your phase diagram. This might happen in a car radiator (with the cap on..assuming you've added no antifreeze), or in a nuclear reactor (the cooling water can be at extreme pressures).
Happens in a pressure cooker, too.
