What do we mean by a dynamic equilibrium? Can you describe how the development of a vapor pressure above a liquid represents such an equilibrium?
1 Answer
Apr 4, 2018
Dynamic equilibrium refers to a state where the forward and reverse reactions are occurring at the same pace. So, although there are still reactions occurring, there is no net change.
An example of dynamic equilibrium is a closed system of a liquid inside of a container (with a lid).
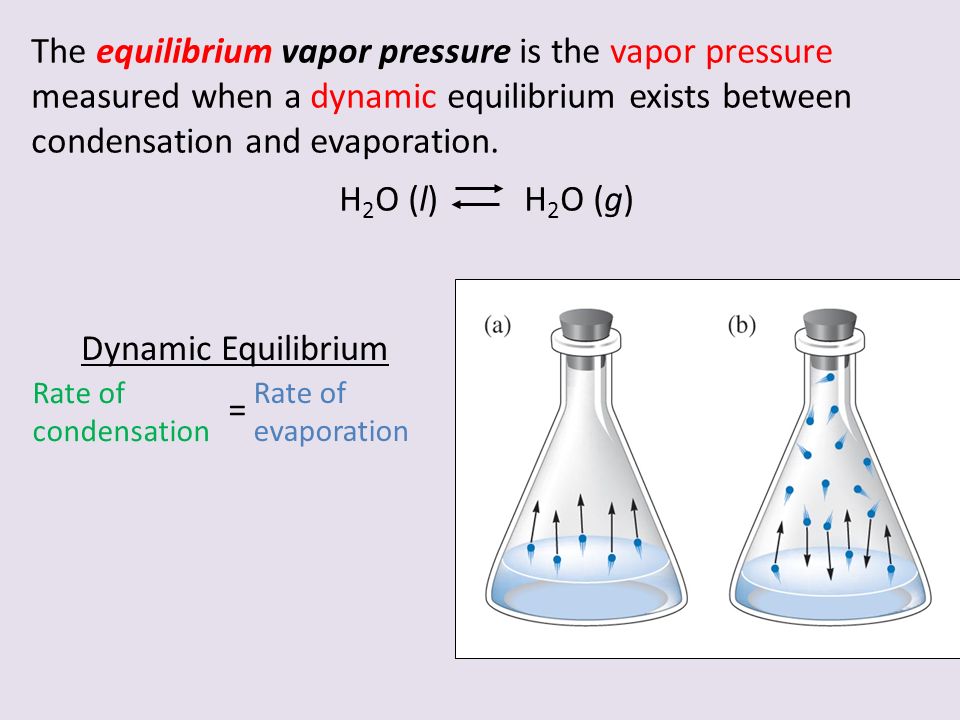
- When the liquid is first placed into the container, it will begin to lose particles to the air above because some particles will be able to escape the liquid phase and move on into the gas phase.
- Particles in the gas phase will also move back into the liquid phase because of the increased vapor pressure.
- Eventually, the rate of these particles moving back into the liquid (known as condensation) will be the same as the rate of gas phase leaving the liquid (known as evaporation).
- When these rates equal, it is known as dynamic equilibrium.

