How do sn1 reactions differ from sn2 reactions?
1 Answer
Apr 10, 2018
Consider the following points of difference.
Explanation:
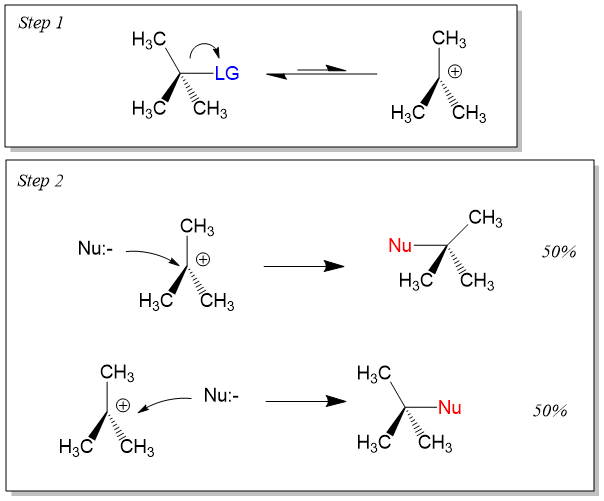
SN1 Reaction
- It is Substitution Nucleophilic Unimolecular reaction.
- Proceeds in two steps.
- First step is the slow one involving dissociation of the substrate to form carbocation intermediate. Second step is the fast one in which the carbocation combines with the attacking Nucleophile.
- More stable carbocation
#rarr# faster is the reaction - It is favored by polar solvents.
- Mostly given by tertiary alkyl halides.
- It occurs with Racemization.
- It follows
#1^(st)# order kinetics. - Rate=k[R-X]
(Image source only)
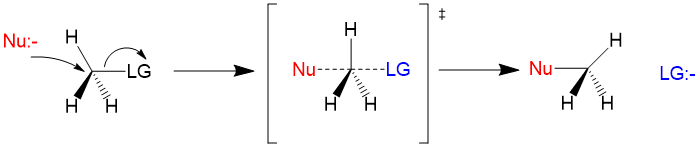
SN2 Reaction
- It is Substitution Nucleophilic Bimolecular reaction.
- Proceeds in a single step which is the rate determining step.
- It is favored by non polar solvents.
- Mostly given by primary alkyl halides.
- Nucleophilic addition takes place from the opposite side of the Leaving group.
- It occurs with 100% inversion of configuration.
- It follows
#2^(nd)# order kinetics. - Rate=k[R-X][Nu]
(Image source only)


