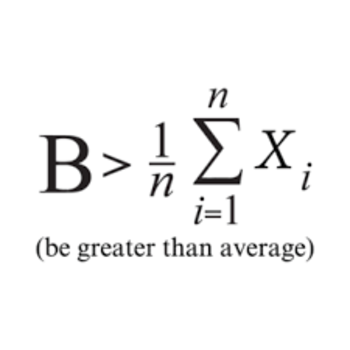Write half reactions for the following equation? : #Mg_((aq))^(+2) + Mn_((s)) -> Mg_((s)) + Mn_((aq))^(+4) #
2 Answers
Writing the Oxidation States for each reactant and product.
We see,
Balancing the charge in this equation,
Also,
Balancing the charge in this equation,
As, we know, Oxidation is losing of electrons and reduction is gaining of electrons, and oxidation occurs at anode and reduction at cathode,
See below:
Explanation:
Divide the equation into the reduction reaction and the oxidation reaction by inspecting the ox-numbers.
But we must equalize the number of electrons on both sides so the half equation is:
So the overall equation is:
And then remove the electrons: