Please explain how can we differentiate beta and alpha-D-fructopyranose from beta and alpha-L-fructofuranose?? What is the difference in their ring structures?
1 Answer
Here's what I get.
Explanation:
The words pyranose and furanose come from the names of two cyclic ethers, pyran and furan.
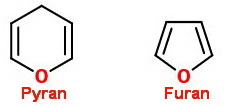
A sugar with a six-membered cyclic ether structure is a pyranose.
A sugar with a five-membered cyclic ether structure is a furanose.
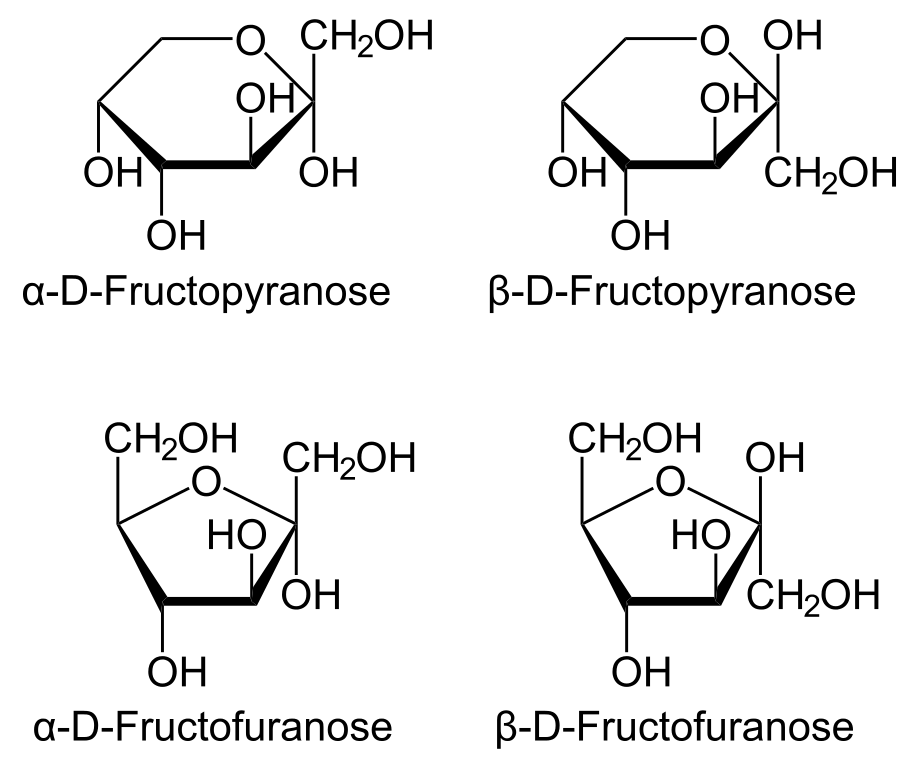
Thus, the two top structures in the diagram are fructopyranoses, while the two at the bottom are fructofuranoses.
The terms alpha and beta refer to the configurations of the anomeric
If the
The L-isomers
The L-isomers of fructose are the corresponding mirror images of the D-isomers.
To convert a D-isomer to its enantiomer, you change the configuration of every chiral carbon.
If a group (
For example, we could say that the configurations of carbons 2 to 5 in α-D-fructofuranose are "down-up-down-up".
In α-L-fructofuranose, the configurations of carbons 2 to 5 are "up-down-up-down", as we see in the picture below.

"We leave it as an exercise for the student" to draw the structures of the other isomers of L-fructose.

