If you want to study the relationship between temperature and pressure of a gas, which factor must be held constant?
2 Answers
Apr 14, 2018
I would say Volume:
Explanation:
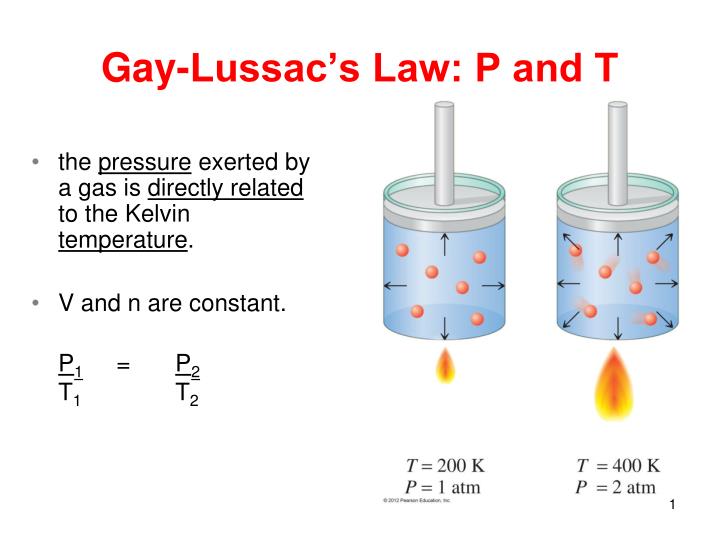
I was thinking of Gay-Lussac Law:
Apr 14, 2018
Volume and number of moles of gas
Explanation:
There exist a relationship between the temperature and pressure of gases. If the volume and the number of moles of gas are kept constant, then the temperature is directly proportional to the pressure of the gas.
This is known as Gay-Lussac's law.
In an equation form, it is written as:
where
If we want to compare the temperature/pressure of a gas after increasing one of its variables, the equation becomes:
#P_1,P_2# are the pressures of the two gases
#T_1,T_2# are the temperatures of the two gases


