What is the order of reactivity towards electrophilic aromatic substitution of fluorobenzene, chlorobenzene and iodobenzene?
1 Answer
Apr 18, 2018
The order of Positive Mesomeric effect
Explanation:
From the order of
Fluorobenzene > Chlorobenzene > Iodobenzene
Hence the reactivity towards electrophilic aromatic substitution follows the same order as given above :
Fluorobenzene > Chlorobenzene > Iodobenzene
The relative rates for nitration are given below :-
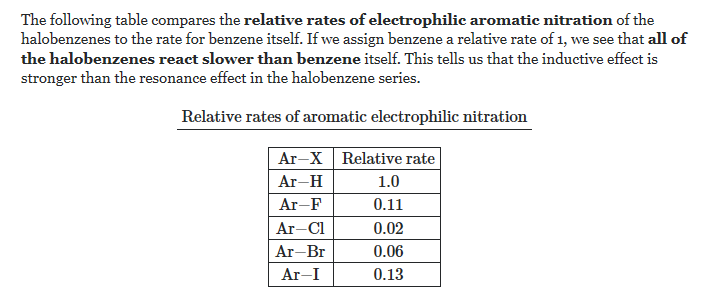 https://chemistry.stackexchange.com/questions/38341/reactivity-of-chlorobenzene-and-benzene-in-electrophilic-substitutions
https://chemistry.stackexchange.com/questions/38341/reactivity-of-chlorobenzene-and-benzene-in-electrophilic-substitutions
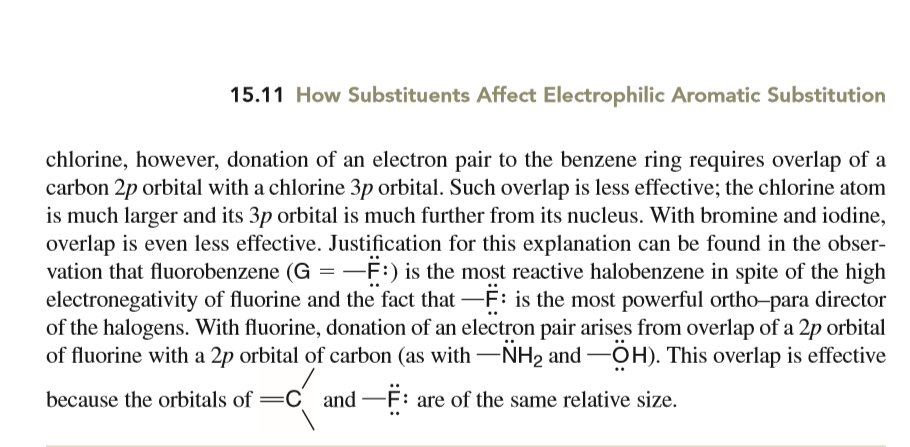 Organic Chemistry book by Solomons 10th edition
Organic Chemistry book by Solomons 10th edition

