Why use phenolpthalein indicator?
In a weak acid strong base titration (tartaric acid with sodium hydroxide) why is phenolphthalein used as the indicator? What is the math reasoning for it, is it anything to do with the Ka values?
In a weak acid strong base titration (tartaric acid with sodium hydroxide) why is phenolphthalein used as the indicator? What is the math reasoning for it, is it anything to do with the Ka values?
1 Answer
Well, (i) because it is convenient, and (ii), it works like a charm....
Explanation:
I usually find phenolphthalein to be the indicator of choice in acid-base titrations (i.e. the base is the titrant). The COLOURED form is the basic form, and in an acid milieu it is COLOURLESS. And the endpoint is VERY easy to recognize, given that it goes from colourless to light pink.
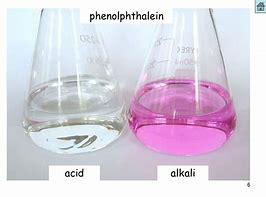
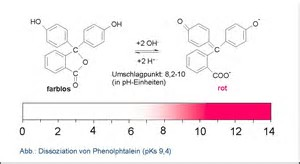
In a titration, we titrate to the endpoint, the point of initial colour change, rather than to the colour change. And the change from colourless to SLIGHTLY pink is easy to vizualize, and non-ambiguous.
The

