The kinetic theory assumes that collisions of gas particles are perfectly elastic. What does this statement mean?
1 Answer
Apr 24, 2018
It means that no energy is gained or lost in these collisions.
Explanation:
In the kinetic molecular theory of gases, this is one of the assumptions for gas particles.
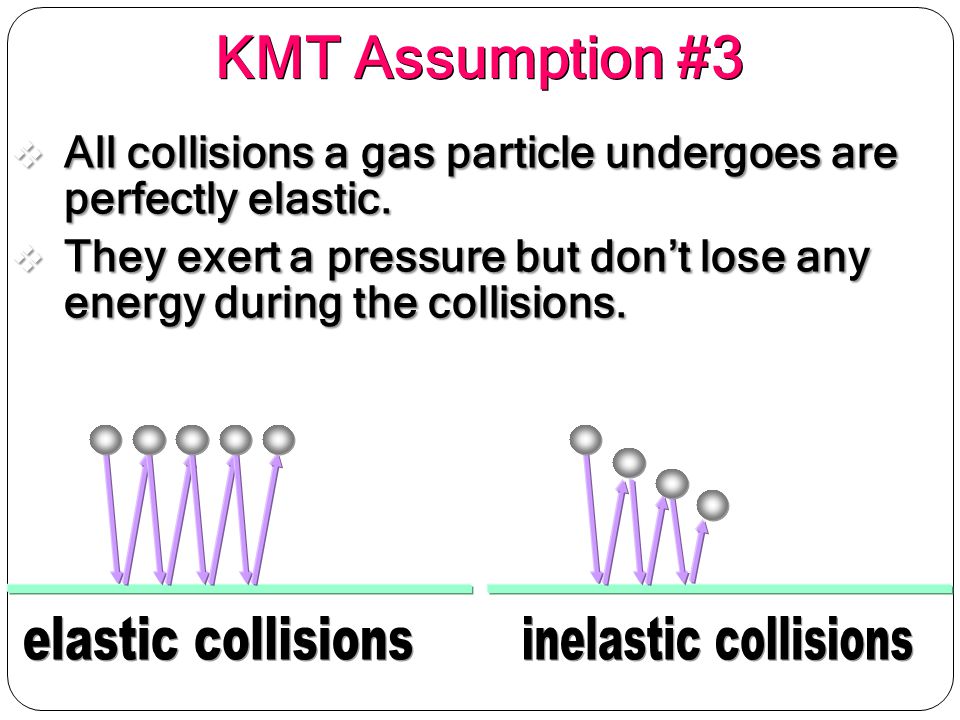
We assume, when gas particles collide with each other or the walls of the container, that no energy is lost during these collisions.
This isn't actually true in real life, though! When gas particles collide in real life, there will be a loss in energy.

