According to Beer’s Law, if the absorbance of a sample is 0.33 at a concentration of 0.20M, what is the absorbance at a concentration of 0.40M?
1 Answer
May 9, 2018
Well, Beer's law gives you a straight line. Would the absorbance not be twice?
If a
Beer's law is given by:
#A = epsilonbc#
and so, if you plot absorbance
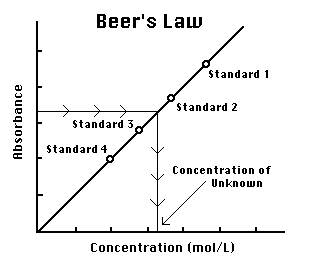
you get the slope
The molar absorptivity is an intrinsic property of the analyte, so it won't change with analyte concentration.
CHALLENGE: From these two data points, calculate the molar absorptivity of the analyte in your solution for a path length of 1 cm.

