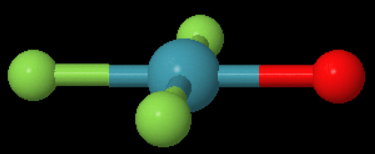
In #"XeF"_3"O"^-#, does the oxygen occupy the axial position in the trigonal bipyramidal or the equatorial position? and why?
1 Answer
May 13, 2018
Here's what I get.
I think you have made a mistake with your Lewis structure.
In
If we give each terminal atom an octet, the central
The Lewis structure is
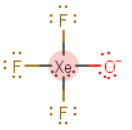
Thus, the electron geometry is octahedral with the lone pairs occupying the axial positions.

The molecular geometry is approximately square planar.