1-aldo-2-methylcyclohex-1-ene is passed through n2h4 in H2O 2 solution. The product is 1-aldo-2-methylcyclohexane with the syn addition of hydrogen atoms. Why the hydrogen atoms are added from the same side?
1 Answer
May 18, 2018
Here's what I find.
Explanation:
The hydrogen peroxide oxidizes the hydrazine to diimide (diazene).
The trans form of diimide is more stable than the cis, but it is the cis form that takes part in the reaction.
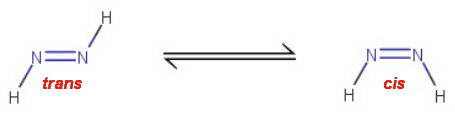
The mechanism involves a cyclic, concerted [4+2] intramolecular addition.
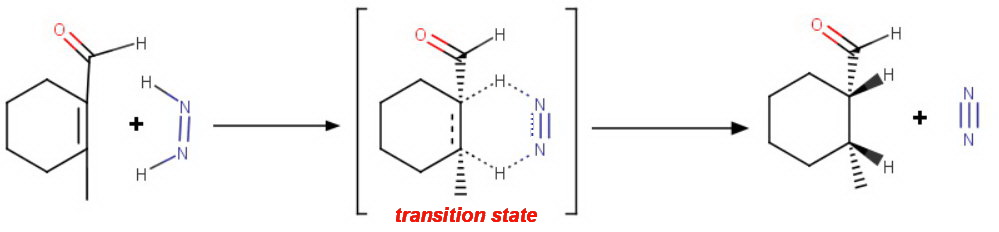
The geometry of the transition state requires the diimide to deliver both hydrogen atoms simultaneously to the same side of the cyclohexene ring (syn addition).
Thus, 2-methylcyclohexenecarbaldehyde forms
cis-2-methylcyclohexanecarbaldehyde.

