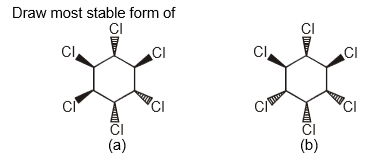
What is the most stable form of the following compounds? (i.e., draw the most stable form in chair form of both the compounds)
2 Answers
Draw in crayon....?
Explanation:
Just to retire this question...you really should have a set of molecular models to address the problem... We know that bulky atoms or groups have unfavourable
Here's what I get.
Explanation:
Part (a)
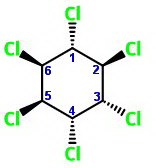
I have oriented the chair forms below so that
The two chair forms are
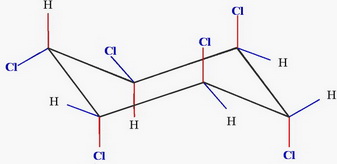
and
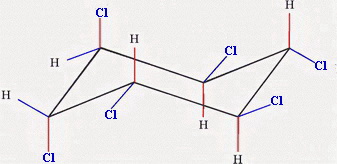
The more stable chair has more of the bulky groups in the equatorial positions.
Chair 1 has two equatorial chlorine atoms, and Chair 2 has four.
Chair 2 is the more stable form.
Part (b)
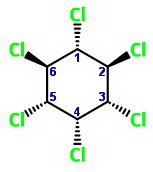
The chair forms are
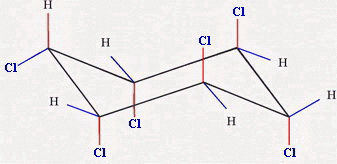
and
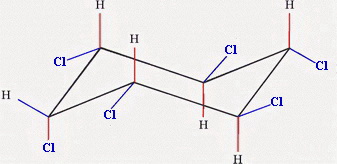
Chair 3 has one equatorial chlorine atom, and Chair 4 has five.
Chair 4 is the more stable form.


