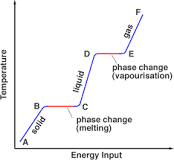
Why does temperature remain constant during fusion or vaporization?
1 Answer
May 21, 2018
Because, the heat energy supplied is used up for the change of phase of matter.
Explanation:
We know that energy is needed for the phase of matter to change.
But, during the phase change, the substance requires an extra amount of heat to melt. This is known as latent heat of fusion. In the same way, when a substance vaporizes, it needs an extra amount of heat energy. This is known as latent heat of vaporization.
The temperature remains constant, because all of the heat energy supplied is used for the change of phase of matter.