How many esters with the molecular formula C_5H_10O_2 can be made by reacting a primary alcohol with a carboxylic acid? A. 4 B. 5 C. 6 D. 8?
1 Answer
May 21, 2018
I count C. six esters.
Explanation:
Methyl esters don't count, because methanol is not a 1° alcohol. It has no alkyl groups attached to the carbon bearing the
There are six esters that satisfy the criteria.
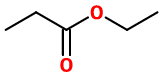 Ethyl propionate
Ethyl propionate
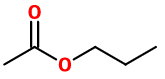 n-Propyl acetate
n-Propyl acetate



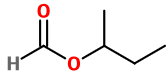 sec-Butylformate
sec-Butylformate

