How do you determine the correct molecular shapes of #GeF_4#, #SeF_4#, and #XeF_4#?
1 Answer
May 22, 2018
By application of VESPER....
Explanation:
We got
And we got
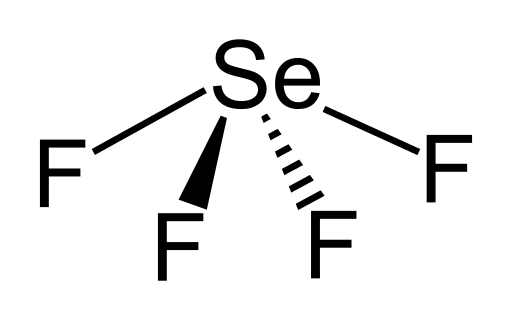
And we
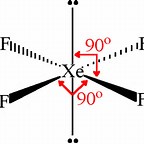
By application of VESPER....
We got
And we got

And we
