What are diamond and graphite?
1 Answer
May 23, 2018
These are
Explanation:
These are both carbon allotropes...of remarkably different appearance. Graphite is non-molecular in two dimensions...
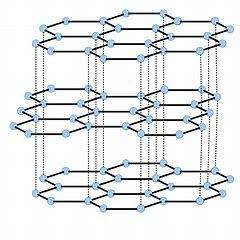
The graphite layers can move with respect to each other, and thus graphite can act as a LUBRICANT....
...whereas diamond is non-molecular in THREE dimensions.
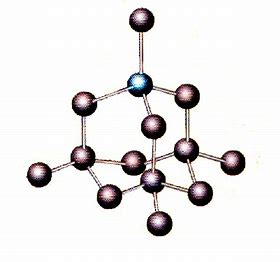
The strength of the carbon-carbon bonds in diamond makes diamond-tipped cutting tools very effective....

