What is observed when adding ammonia to Cu2+ solution, and why does this happen?
1 Answer
May 29, 2018
A blue solution will form.
Explanation:
The reaction between copper(II) ions and aqueous ammonia will create a beautiful blue color of aqueous copper(II) ions.
An example would be to combine copper(II) sulfate and ammonia solution. The equation for this reaction is:
The blue color comes from the copper(II) complex ions, such as
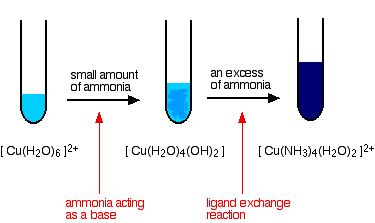
When an excess of ammonia is added, you get a dark-blue color. This happens when ammonia becomes a ligand and surrounds the copper ion. The equation then becomes:

