How do you write the equation for this reaction: Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas?
1 Answer
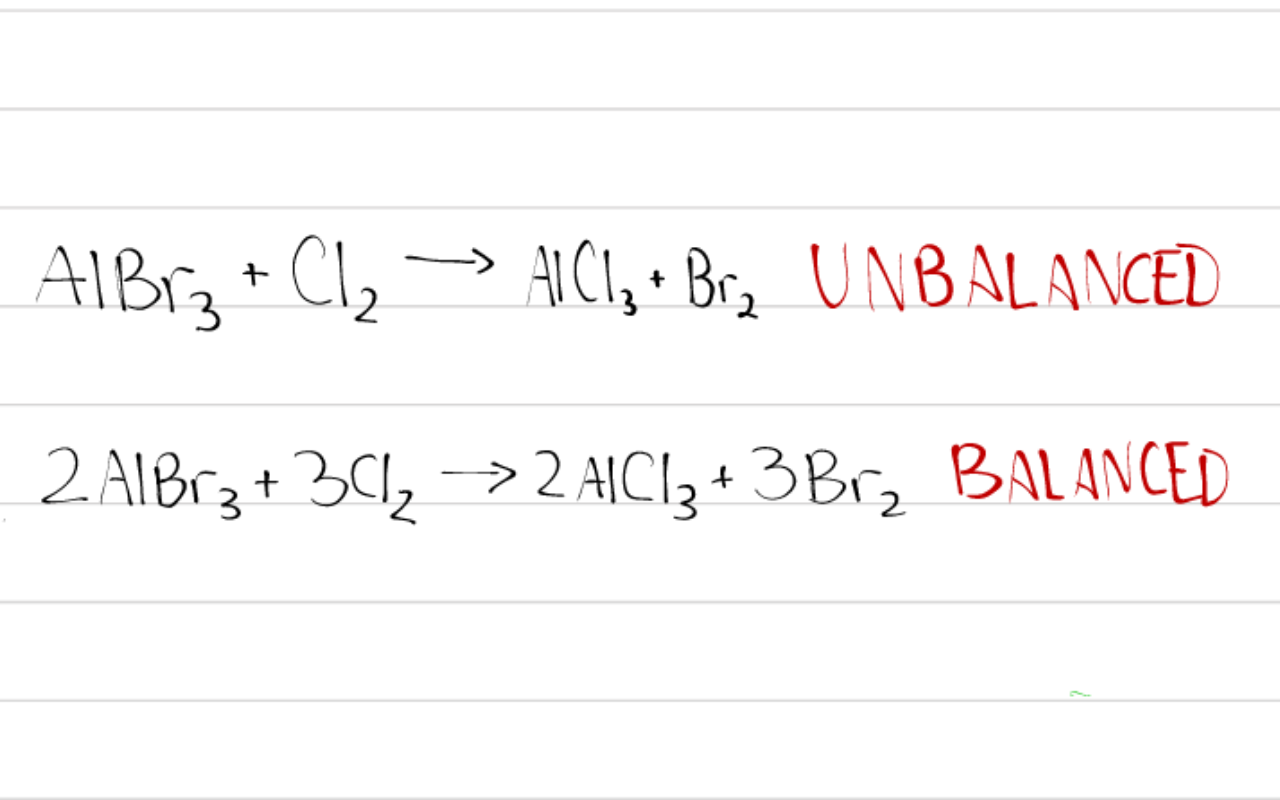
Explanation:
In compounds, the oxidation numbers must add together to become
In aluminum bromide, aluminum has an oxidation number of
This is why one reactant is
The other reactant,
This is a single replacement reaction, in which the free element in the reactants becomes part of the compound in the products, replacing one of the original elements in the compound, and makes the replaced element become the free element in the products.
Bromine is the replaced element and becomes
The oxidation number of chlorine is
The second equation given is balanced by mass, but if you don't need a balanced equation, use the first equation.


