What is the C oxidation state, O oxidation state, and the H oxidation state?
1 Answer
Jun 1, 2018
Refer below.
Explanation:
In
In general,
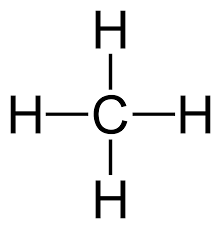
- CARBON: Oxidation state of carbon can vary widely, from
-4 (inCH_4 ) to+4 (such as inCO_2 ). - OXYGEN: The oxidation state of oxygen in its compounds is -2, except for peroxides like
H_2O_2 , andNa_2O_2 , in which the oxidation state forO is-1 . - HYDROGEN: The oxidation state of hydrogen is
+1 in its compounds, except for metal hydrides, such asNaH ,LiH , etc., in which the oxidation state for H is-1 .
