What's the formula for potassium sulfate?
1 Answer
Jun 5, 2018
Explanation:
Potassium:
Sulfate:
We can't just combine them as it is, since potassium and sulfate have different charges. We need to equalize charges by criss-crossing the charges, like this example:
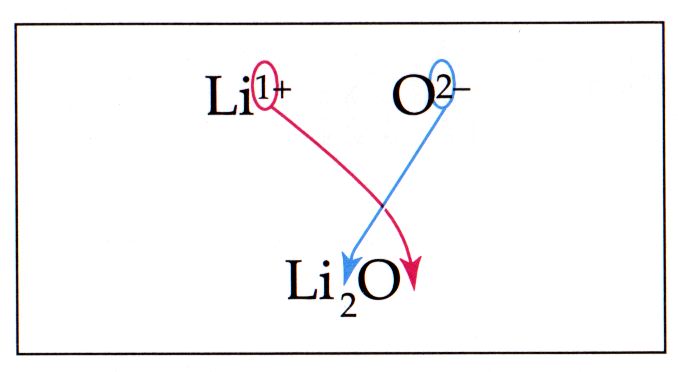
Following this image, we know that since potassium has just 1 positive charge , then there is just 1 molecule of sulfate.
Also, sulfate has a negative 2 charge, so there will be 2 atoms of potassium.
This can be written out as:
Hope this helps!

