Molecules in order of decreasing dipole-dipole strength?
HF
HCI
HBr
HI
HF
HCI
HBr
HI
1 Answer
Jun 6, 2018
Explanation:
- In a polar molecule, one atom will be more electronegative, or be better at hogging electrons in the bond.
- So, electrons will spend more time with the more electronegative atom.
- This causes the side of the molecule with the more electronegative atom to become slightly negatively charged, and the other side to become slightly positively charged.
- The slightly positive side of one molecule will attract to the slightly negative side of another molecule.
These attractions are known as dipole-dipole forces.
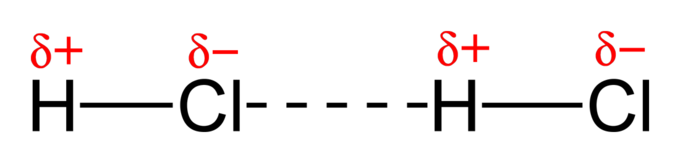
To determine the relative strengths of dipole-dipole forces, we have to look at differences in electronegativity.
If we have a high difference in electronegativity between the atoms in a molecule, the slightly negative end will be more negative, and the slightly positive end will be more positive.
This means that the magnitude of the attraction between the slightly positive and negative ends will increase—leading to stronger dipole-dipole forces.
In our case, we have hydrogen paired up with four different halogens:
Here are their positions on the periodic table:
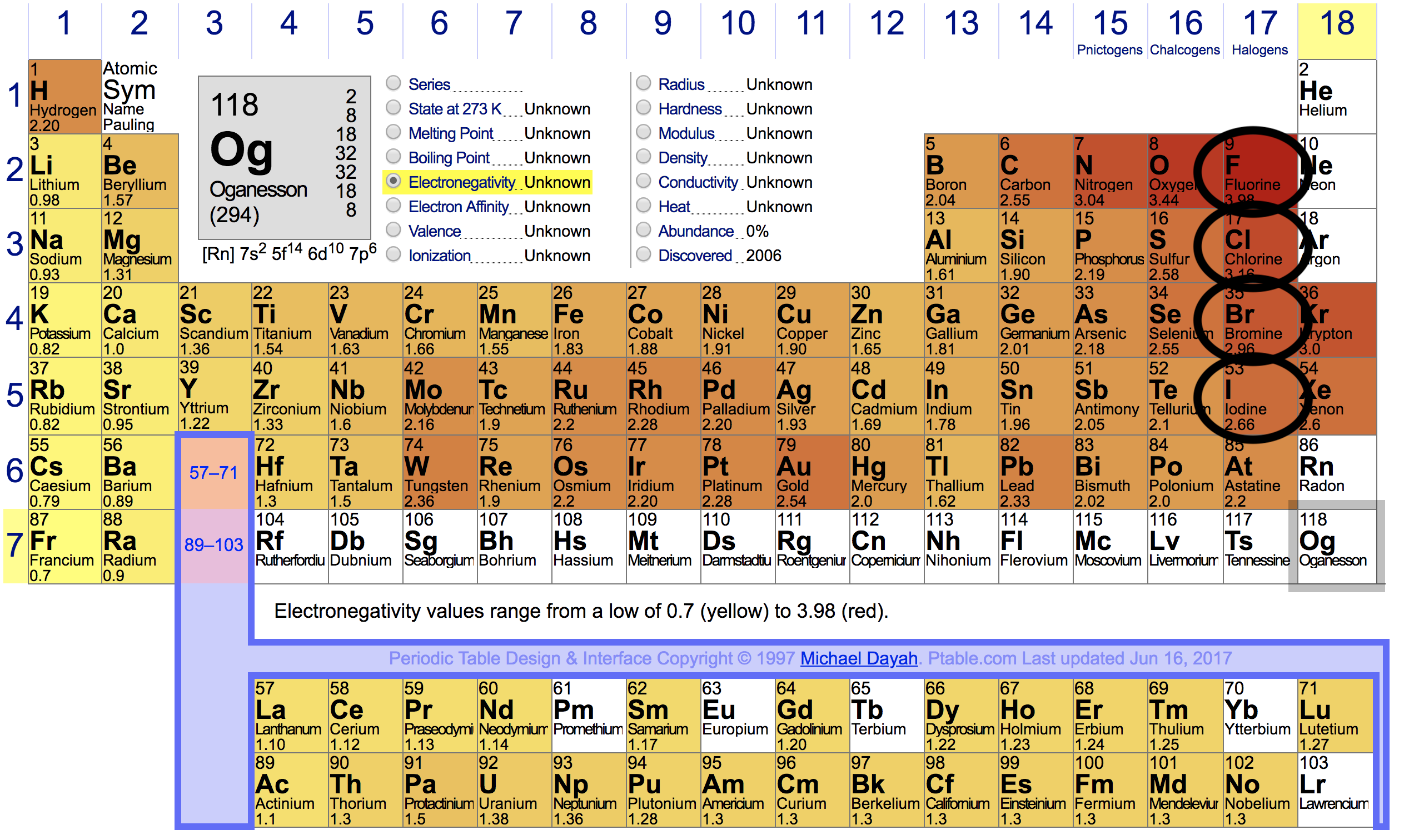
From this, we can see that:
- The electronegativity difference between
#F# and#H# in#HF# is the greatest. This means that dipole-dipole forces between#HF# molecules is the strongest. - The electronegative difference between
#H# and#Cl# is less than#H# and#F# , but more than#H# and#Br# . So, the strength of dipole-dipole forces in#HCl# will be between#HF# and#HBr# . - The electronegative difference between
#H# and#Br# is less than#H# and#Cl# , but more than#H# and#I# . So, the strength of dipole-dipole forces in#HBr# will be between#HCl# and#HI# . - The electronegative difference between
#H# and#I# is the lowest, so its dipole-dipole forces are the weakest.

