Table salt #"NaCl"# dissolves well in water whereas sand- a mixture containing primarily quartz #"SiO"_2#- does not. The difference in the solubility of the two species enables their separation through filtration.
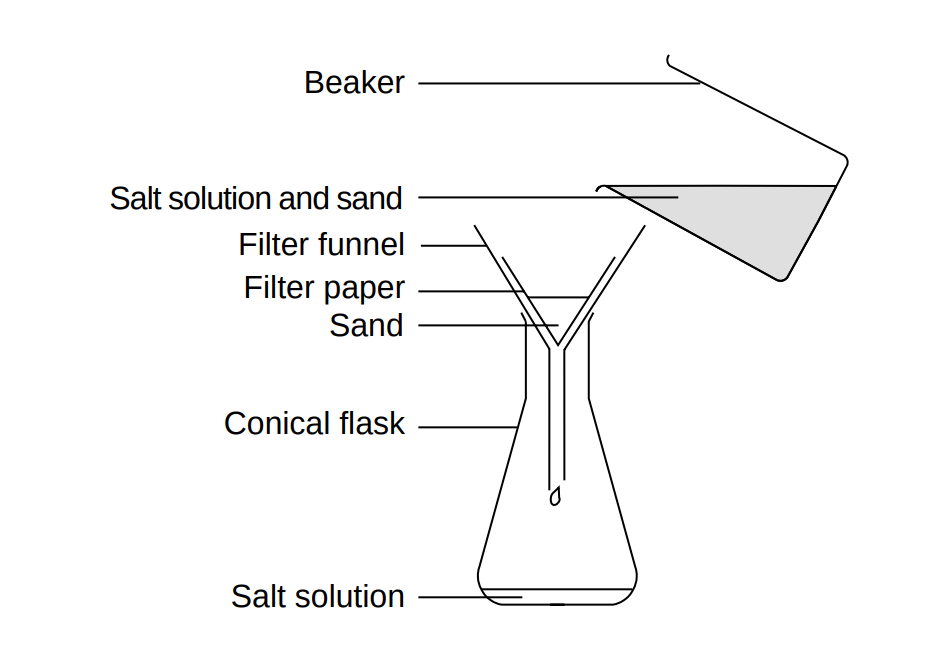
For example, to separate a mixture of sand and table salt, start by dissolving the solid mixture in water to form a #"NaCl"# solution that contains sand. The solution part shall pass through a piece of filter paper whereas sand will be trapped on the funnel. Evaporating the salt solution removes #"H"_2"O"# such that #"NaCl"# is restored to its crystal form.
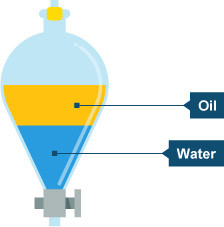
Separation funnels (see diagram below) facilitates the separation of two immiscible liquids - liquids that don't dissolve in each other e.g., water and mineral oil.
What if the two liquids dissolve in each other in significant amounts? Fractional distillation separates substances of different boiling points. For example, it is possible to isolate ethanol #"C"_2"H"_5"OH"# from its solution in water #"H"_2"O"#- the two liquids mix at any ratio due to their capabilities of forming hydrogen bonds- with the help of distillation given that ethanol boils at a temperature lower than that of water.
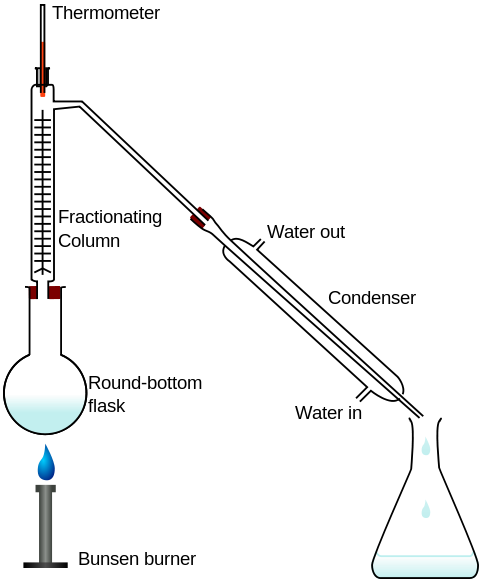
In the particular setup shown in the diagram, the temperature of the mixture in the round-bottom flask is maintained between #78.37color(white)(l)°"C"# the boiling point of ethanol and #100color(white)(l)°"C"# the boiling point of water, such that ethanol vaporizes, leaves the mixture, condenses, and collects in the conical flask whereas water remains in the mixture.




